i.Mune® TBNK [CE]
(not available in the United States of America)

Now available as CE-IVD test for dried blood spot (DBS) samples!
i.Mune TBNK [CE] is a quantitative in vitro test to determine the percentages and absolute counts of human lymphocyte subsets in liquid venous whole blood and to determine the percentages of human lymphocyte subsets in capillary whole blood specimens dried on filter paper (Dried Blood Spot; DBS).
Benefits
i.Mune TBNK [CE] enables the epigenetic quantification of:
- T lymphocytes (CD3+)
- B lymphocytes (CD19+)
- Natural killer lymphocytes (CD16+CD56dim) (Natural killer cells)
- Helper T lymphocytes (CD3+CD4+)
- Cytotoxic T lymphocytes (CD3+CD8+).

Contents
i.Mune TBNK [CE] is comprised of the three (3) separate kits:
- i.Mune Prep (for preparation of a total of 48 samples)
- i.Mune TBNK Amp (for PCR-amplification of 48 samples)
- i.Mune Check (controls and standards for a total of 12 runs)
In addition, we'll provide a Data Analysis Tool (MS-Excel based)
Sample Requirements

- Capillary whole blood dried on filter paper
-
- Samples can be stored at room temperature (15°C to 30°C) or frozen (-30°C to -15°C) for up to 12 weeks
OR
- 40 µl of liquid venous whole blood collected in K2EDTA blood collection tube
-
- Samples can be stored at room temperature (15°C to 30°C) for up to 24 h
Applications
According to published data, quantification of T-/B- and NK lymphocytes can be useful for*:
- Follow-up and diagnostic evaluation of primary immunodeficiency 1),2)
- Monitoring of HIV-positive patients 1),3)
- Immune monitoring following immunosuppressive therapy for transplantation, autoimmunity and other immunological conditions4)
- Assessment of immune reconstitution post hematopoietic stem cell transplantation5)
- Absolute quantification of circulating B cells for diagnosis of chronic lymphocytic leukemia (CLL) patients6)
Please inquire for a customized proposal:
*The above clinical applications have been established using technologies currently being employed in clinical laboratory routine (e.g. flow cytometry).
Performance Characteristics:
Performance characteristics of i.Mune TBNK [CE] were determined according to CLSI guidelines.
| Repeatability (single-site) | CV < 12,5% (liquid venous whole blood); CV < 25% (DBS) |
| Within Laboratory Precision (single-site) |
CV < 25% (both liquid venous whole blood and DBS) |
| Reproducibility (multi-site) |
CV < 25% (liquid venous whole blood) |
| Within Laboratory Precision (multi-site) |
CV < 25% (liquid venous whole blood) |
| Linearity |
10 - 40 µl (liquid venous whole blood) |
| Limit of Quantification |
< 100 copies (liquid venous whole blod and DBS) |
| Reliability |
99% (liquid venous whole blood and DBS) |
| Interference |
No interference with bilirubin (0,4 mg/ml), |
Comparison i.Mune TBNK [CE] with flow cytometry
113 liquid venous whole blood samples from self-declared healthy donors were prospectively collected in K2EDTA blood collection tubes. Epigenetic data of 112 test samples analyzed with i.Mune™ TBNK were compared to flow cytometric data using the Spearman correlation coefficient (Spearman r) for each assay (% and cells/µl).
| Assay | Spearman r | p value |
| CD3 cells/µl | 0.82 | <0.0001 |
| CD4 cells/µl | 0.70 | <0.0001 |
| CD8 cells/µl | 0.55 | <0.0001 |
| BLC cells/µl | 0.79 | <0.0001 |
| NKC cells/µl | 0.70 | <0.0001 |
| Leukocytes/µl | 0.70 | <0.0001 |
| CD3 % of Leukocytes | 0.85 | <0.0001 |
| CD4 % of Leukocytes | 0.76 | <0.0001 |
| CD8 % of Leukocytes | 0.53 | <0.0001 |
| BLC % of Leukocytes | 0.86 | <0.0001 |
| NKC % of Leukocytes | 0.78 | <0.0001 |
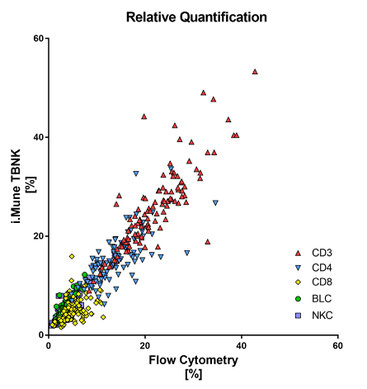
r = 0.90
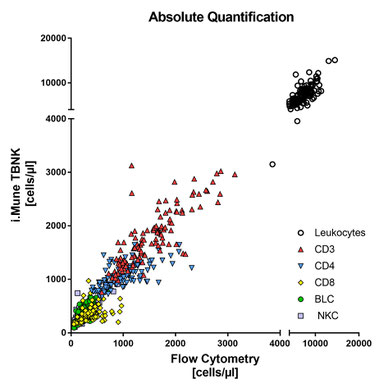
r = 0.96
Reference Ranges (liquid whole blood vs. dried blood)
112 liquid venous blood samples and 108 capillary whole blood samples were prospectively collected from self-declared healthy subjects (age 18 to 71; female and male) and analyzed using i.Mune TBNK [CE]. Reference ranges for the individual lymphocyte subsets were defined using the lower and upper limit for each cell type applying a 2.5 to 97.5 percentile. The figure shows the comparison between liquid venous whole blood and DBS samples.

References
- Baron U. et al. Epigenetic immune cell counting in human blood samples for immunodiagnostics. Sci Transl Med. 2018 Aug 1; 10 (452)
- Aluri J, et al. Clinical, Immunological, and Molecular Findings in 57 Patients With Severe Combined Immunodeficiency (SCID) From India. Front Immunol. 2019 Feb 4;10:23
- Ford N, et al. The evolving role of CD4 cell counts in HIV care. Curr Opin HIV AIDS. 2017 Mar;12(2):123-128
- Omana-Zapata I, et al. Accurate and reproducible enumeration of T-, B-, and NK lymphocytes using the BD FACSLyric 10-color system: A multisite clinical evaluation. PLoS One. 2019 Jan 28;14(1):e0211207
- Riley RS. Laboratory evaluation of the cellular immune system. In: McPherson RA, Pincus MR, eds. Henry's Clinical Diagnosis and Management by Laboratory Methods. 23rd ed. St Louis, MO: Elsevier; 2017:chap 45
- Hallek M, et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008 Jun 15;111(12):5446-56

